[반응공학] 암모니아 합성(Ammonia synthesis)영문
 등록일 / 수정일
등록일 / 수정일 페이지 / 형식
페이지 / 형식 자료평가
자료평가 구매가격
구매가격
- 2009.08.27 / 2019.12.24
- 15페이지 /
 hwp (아래아한글2002)
hwp (아래아한글2002) - 평가한 분이 없습니다. (구매금액의 3%지급)
- 1,400원
최대 20페이지까지 미리보기 서비스를 제공합니다.
자료평가하면 구매금액의 3%지급!
 1
1 2
2 3
3 4
4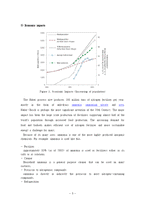 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15
추천 연관자료
- 목차
-
1. Introduction
2. A Brief of History of Ammonia Synthesis
3. The Societal Need and Economic Impacts
4. A Brief Description of
Ammonia synthesis process
5. Kinetics, Performance Equations,
the Contacting Patterns related to the process.
6. Reference
- 본문내용
-
1. Introduction
Ammonia (NH3) is one of the most familiar compounds of nitrogen and hydrogen as well as one of the world's most valuable industrial and agricultural chemicals. Ammonia is used throughout the industrial world as a valuable fertilizer.
Ammonia and its compounds, primarily ammonium nitrate and other ammonium salts, replenish nitrogen in depleted soils. This greatly increases yields of agricultural crops, especially those that cannot obtain nitrogen from the atmosphere. The ammonia is converted to nitric acid, an essential ingredient in explosives, via the Ostwald-Bauer process invented by German chemist Wilhelm Ostwald (1853-1932) in 1901. During World War I, synthetic ammonia was used to manufacture ammunition after Germany's nitrate supplies had been cut off.
Commercial ammonia synthesis takes place in large steel reactors that are designed to withstand very high pressures (up to one thousand atmospheres) and high temperatures (about 700°C or 1300°F). The reactors also resist attack by hydrogen gases which can corrode many metals, especially at high temperature and pressure. The catalyst in commercial ammonia synthesis is usually an iron oxide mixed with a small amount of additional "promoter" material. When the process begins, the hydrogen gas converts the catalyst to pure iron. Gradually, as the iron catalyst is poisoned by carbon and sulfur compounds, it becomes less effective and must be replaced. Nitrogen gas for the synthesis process comes from the atmosphere, while most of the hydrogen needed is extracted from natural gas sources. Once ammonia gas has been formed, it is liquefied by cooling it with water. Nitrogen and hydrogen that have not yet been converted to ammonia are recycled through the system. In addition to Haber-Bosch synthesis, other industrial sources of ammonia also exist; it is created as a by-product, for example, during the production of coal and coke-oven gas.
In the early 1990's the M.W. Kellogg Co. developed a new reduced energy process for manufacturing ammonia. This technology was successfully implemented by Pacific Ammonia Inc at its Kitimat, British Columbia plant in 1994. This new technique can increase ammonia production by 40% by using two innovative processes. The first innovation, known as the Kellogg Advanced Ammonia Process, involves a ruthenium-based catalyst instead of a traditional iron catalyst. The second innovation, the Kellogg Reforming Exchanger System, uses a new open-tube design in the reforming exchanger.
2. A Brief of History of Ammonia Synthesis
In ancient times, ammonia was derived from organic material such as manure and urine. During the Middle Ages, alchemists produced a weak solution of ammonia in water by distilling the horns and hoofs of oxen. They called this ammonia solution "spirits of hartshorn." Later, more substantial amounts of ammonia were recovered from the vapors of natural steam vents in Italy, and large quantities of ammonia were produced from Chile saltpeter (sodium nitrate, or NaNO3), which is mined in South American deserts. Around the turn of the twentieth century, Sir William Crookes predicted that the world would soon run short of Chile saltpeter. Scientists feared that without nitrogen-based fertilizers to increase crop yields, the growing world population would starve. Thus began the search for an alternative source of ammonia. Although a practically inexhaustible source of nitrogen exists in the atmosphere, which consists of about 80 percent nitrogen, no one knew how to convert the element into ammonia on a large scale. In Germany, Fritz Haber began investigating the possibility of combining nitrogen from the atmosphere with hydrogen to form ammonia. In principle the chemistry of ammonia synthesis was simple but the execution was difficult because high temperatures and very high pressures were needed to produce enough quantities of ammonia to make the process economical. Habersucceeded in developing an ammonia synthesis process that worked in the laboratory in 1909. Initially, Haber used osmium or uranium as a catalyst to speed up
- 참고문헌
-
① Jayant. M. Modak, Harber Process for Ammonia Synthesis, Resonance, 2002
② A. Ozaki and Hugh Taylor, Kinetics and mechanism of Ammonia Synthesis, Proceeding of the Royal Society of London, 1960
③ J. Morud, "The dynamics of Chemical Reactors Whit Heat Integration", Ph.D thesis, 1995.
④ S. S. Elnashaie, M. E. Abashar And A. S. Al. Ubaid, "Simulation and Optimization of an Industrial Ammonia Reactor", Ind. Eng, chem.. Res, Vol. 27,pp. 2015, 1988.
⑤ D.C. Dyson and J.M. Sicon. "A Kinetic Expression With Diffusion Correction for Ammonia
⑥ Synthesis on Industrial Catalyst", Ind. Eng. Chem. Fundamental, Vol. 7, No. 4, pp. 605, 1986.
⑦ P.P. Singh and N. Saraf "Simulation of Ammonia Synthesis Reactor", Ind. Eng. Chem. Process Des. Dev, Vol. 18, No.3, pp. 304, 1979.
자료평가
-
아직 평가한 내용이 없습니다.
오늘 본 자료
더보기

최근 판매 자료
- 물리화학 보고서- Conjugation 염료의 흡수 스펙트럼
- 일반물리학 실험 - 일과 에너지 결과보고서
- 디지털 회로 실험 및 설계 - ADC(Analog to Digital Converter) 실험 1
- [도로교통] 도로 폭에 따른 구간 내 LOS 분석
- [나노기술]나노기술의 정의와 응용분야 및 발전방향(A+레포트)★★★★★
- [도로교통] 도로 폭에 따른 구간 내 LOS 분석
- [창의공학] 계단 오르는 유모차 문제점 및 해결방안
- 모발 염색의 종류와 원리
- [조경실무]조경실무 - 도시경관의 문제점과 발전방향
- 바이오인포매틱스(생물정보학)개념, 바이오인포매틱스(생물정보학)정보과학적기법, 바이오인포매틱스(생물정보학)현황, 바이오인포매틱스 과제
저작권 관련 사항 정보 및 게시물 내용의 진실성에 대하여 레포트샵은 보증하지 아니하며, 해당 정보 및 게시물의 저작권과 기타 법적 책임은 자료 등록자에게 있습니다. 위 정보 및 게시물 내용의 불법적 이용, 무단 전재·배포는 금지됩니다. 저작권침해, 명예훼손 등 분쟁요소 발견시 고객센터에 신고해 주시기 바랍니다.









